Classification :
|
Dana
No: 9.2.1.1
Hey's CIM Ref.: 8.4.7 |
Strunz
: III/A.08-10 III - Halogenures III/A - Halogenures simples: Halogen = 1 : 2, 1 : 3 III/A.08 - Fluorite series and related compounds III/A.08-10 Fluorite CaF2 F m3m 4/m 3 2/m III/A.08-20 Frankdicksonite BaF2 F m3m 4/m 3 2/m III/A.08-25 Haleniusite-(La)! (La,Ce)OF F m3m 4/m 3 2/m III/A.08-30 Tveitite-(Y) Ca1-xYxF2x,x~0.3 Unk Mono III/A.08-40 Gagarinite-(Y) NaCaY(F,Cl)6 P 3 3 III/A.08-45 Zajacite-(Ce) Na(REExCa1-x)(REEyCa1-y)F6where(xney) P 3 3 III/A.08-50 Laurelite Pb7F12Cl2 P 6 6 III/A.08-60 Coccinite* HgI2(?) P 41/nmc 4/m 2/m 2/m |
Historique:
Year of the discovery: 1529
Etymology
: from the Latin "fluere" = to run out, it is an allusion to its metallurgy
property of helping the scoria to flow. The term "fluorescent"
comes from the name "fluorite" which often shows this property.
The nam of the element 'fluor" comes from this mineral, too.
Notice that the term "fluorine" is used in French to be a synonym
of "fluorite". In English, "fluorine" is designs the
element F (7th column of the Medeleiev classification).
Names
:
| *
Androdamant * Antozonit (Stinkspat) * Blue John ( du Français "bleu-jaune") * Bruiachit (Magadam, 1886) - Bruiachita, Bruiachite * Calx fluorata * Cam - Cam Cand * Chaux fluatée (Franz., Hauy, 1801) * Chlorophan * Choneuticit *Chrome-Fluorite * Derbyshire Spar * Espato fluor (Esp.) * Fetid Fluor * Fluate de chaux * Fluor * Fluor mineralis Stolbergicus * Fluorbaryt (Hausmann, 1847) - Fluorbaryte * Fluores (Boetius de Boodt, 1609) * Fluorit * fluorite (international) * Fluorine (France.) * Fluorite (Angl.) * Fluor spar (Angl.) * Fluss * Flußspat - industrie * Flussspat -(Allemagne) |
*
Flußspat
Glas-Spat Kand Liparite (of Glocker) * Fluorina (Italie) * Fluorita (Espagne) * Fluorure de calcium * Flussaures * Glasspat (Cronstedt, 1778) * Gunnisonit (Clarke & Perry, 1882) * Hüttenspat * Kalk Flusse * Keramikspat * Linsenspat * Lithophosphorus Suhlensis * Lysspat * Murrhina * Optischer Spat * Ratofkit (sediment. Fluorit v. Fluss Ratofka, Russie) * Pyrosmaragd * Pseudonecerit * Säurespat * Smaragdfluss * Spath fusible * Spath vitreux -Spatum vitreum * Spato fluore * South African Emerald * Stinkfluss |
- spath-fluor and fluorspar: commercial and industrial words; it indicates that a product contains a certain ratio of CaF2.
Varieties :
- "
Blue John " : purple and white gathered, purple and yellow.
- chlorophane : thermoluminescent variety, wich gives a light green.
- yttrofluorite : the yttrium element sustitutes partially the calcium;
formula : (Ca, Y)F2
- yttrocerite : partial substitution of the Ca by cerium and yttrium;
formula : (Ca, Ce, Y)F2.
- antozonite : deep purple (violet) variety which contains fluorides ions
(F-) non linked. A strong ozone (O3) smell appears after the breack. This
fluorite is found inthe uranium sites.
- African esmerald, false amethyst are "exotic" names ...
La fluorine est communément trouvée en tant que gangue dans les veines hydrothermales, particulièrement celles contentant des mineraux du zinc et du plomb. Elle est également présente dans des greisens, granites, des veines de haute température, dans des marbres et d'autres roches metamorphiques.
Chemical
class : HALIDES
Chemical subclass : simple halides
Chemical formula : CaF2 (calcium fluoride)
Impurities, traces: Y;Ce;Si;Al;Fe;Mg;Eu;Sm;O;Organic matter;Cl;REE (rare
earth elements)... and other;
Crystallographic
properties
Crystalline system : CUBIC
Class symmetry : 4/m -3 2/m cubic Holohedrism
Network : centered faces F
a: 5,46
Z: number of chemical formulas per unit of cell: 4
Morphology : cubic, octahedral; dodecahedral; solid mass; granularity, coarse; fine grained; botryoidal, fibrous; columnar; earthy; aggregate; hexaedric; cuboctaedric; concretionary.
Color
: from colorless to all colors. Several colours can also exist.
It should be noted that the black fluorite (which is not a color!) is
not in nature.
There is only purple dark fluorite, a color similar to black.
Aspect in-situ : crystalline masses of glare vitreous, often cleavable, and in fibro-lamellate masses of sintered structure.
Stripe : white.
Powders
: white (except for certain dark fluorites which after crushing give
a purplish powder,
or pink).
Hardness : 4 (on the Mohs hardness scale)
Index
of refraction : N = 1.434
A low birefringence and interior groupings showing a noncubic symmetry
are also found.
Density : 3.01 to 3.25 - average = 3.13
Point melting : 1360 °C
Crystalline
forms :
- cubic (6 square faces);
- truncated cubic (truncation of the tops of the cube);
- octahedral regular (8 faces in equilateral triangles);
- rhombododecahedron, or rhomboidal dodecahedron(12 faces);
- trigonotrioctahedron (24 faces: 3 faces on each face of octahedral known
as pyramidal).
The fluorite is also in sintered, granular or compact cleavable masses.
| Twins : twins by rotation around a ternary axis, two interpolated cubes. |
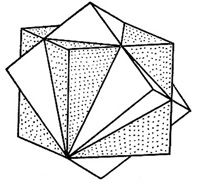 |
Transparency : all optical qualities are found: gem with the stony one.
Glare : vitreous.
Light
: - vitreous glare, given lustre to a little on a1;
- the fluorite is more permeable to UV than quartz.
Cleavage : octahedral; perfect (dominating character).
Break : subconchoidale with unequal; irregular.
Tenacity : fragile, breakable.
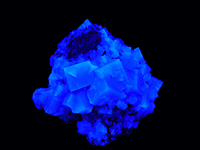
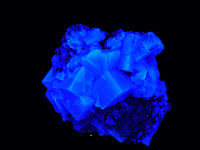
Fluorescence
: - long waves UV(365 Nm): purple
- short waves UV(254 Nm): blue
Other colors can be observed; they depend on the nature of inclusions
(yellow, red...).
|
FLUORITE,
Weardale, Durham, Great Britain
Daylight |

|
|
Long
wave UV
|
|
|
FLUORITE,
Villabona, Spain
Daylight |

|
|
Long wave UV collection and photography © Patrick Arweiler |
|
 |
 |
 |
FLUORITE, Rogerley, Frosterley, Durham, Angleterre UV
longs / long-wave |
Triboluminescence : yes
Heat
Action : - thermoluminescence (chlorophane is the name given to the
varieties that take a green colour).
- decrepitates quickly.
- blowtorch: melts easily and gives a white enamel, with alkaline reaction.
Solubility
: - in the sulphuric acid (H2SO4), hydrofluoric acid (HF), it gives
for the development of all the fluorocarbonaceous compounds;
- slightly soluble in the hydrochloric acid (HCl), insoluble in HF;
- weak solubility in pure water (16 mg/L with 18°C);
- more important in hot water of alkaline nature
(the fluorine content of mineral waters is related to their temperature).
Chemical : Ca: 51,33 %, F: 48,67%
Molar
mass: 78,0748 g/mol
Varieties : - "Blue John": after heating joined together crimson
and white, crimson and yellow.
- chlorophane: variety which is thermoluminescent in clear green.
- yttrofluorite: yttrium sustitutes partially calcium; formulate: (Ca,
Y)F2
- yttrocerite: partial substitution of Ca by cerium and yttrium; formulation:
(Ca, Ce, Y)F2.
- antozonite: dark violet variety which contains independant fluorine
ions (F -). A strong ozone smell emerges from some of them after break
; Some antozonite may occurin the uranium-bearing fluorite lodes.
Pseudomorphosis : often from quartz, chalcedony, and sometimes from siderite (Peyrebrune)
Inclusions
: - solids: pyrite, chalcopyrite, galena, barite...
- liquids: hydrocarbons, various organic matter...
Use : metallurgy, chemical industry, ceramics and glassmakings, abrasive, industry of cements, optics, decoration, medical field...
Gitology
: formation at low temperature. Special abundance in France.
- in the pneumatolytic conditions, associated cassiterite, topaz, lepidolit,
tourmaline of some greisens, aquamarine...
- in hydrothermal conditions as gangue of many ore deposits with galena,
sphalerite, barite, quartz or as principal component of certain primarily
fluorited ore deposits;
- in sedimentary rocks.
In France,
the magmatic influence being probable in some cases but not evident, special
empahsis has been put by the scientists to distinguish objectively, among
the fluorite deposits, veins crosscutting the stratification boundaries,
and stratabound mineralisations rather disseminated or fissural inside
one sedimentary layer.
The former are examplified by the Valzergues mine; the latter by the large
occurences of Morvan area and Burfondy. The purists distinguish as well
two classes:
-ore veins hosted (totally or partially) by the paleozoic crystalline
basement (=basement veins generally linked with great distension faults
or graben tectonics, like in Maure-Esterel, or compression transverse
faults like in the Tarn)
-veins entirely hosted by the sedimentary mesozoic overburden (=cover
veins, the type of which is better examplified by lead-zinc veins of Diois,
Baronnies ans Ardeche than by fluorite veins).
The Illinois-Kentucky fluorite deposits (Cave-in-Rock type) and the Pennine deposits are typical of a more particular type (called Mississipi Valley type) characterized by karstic pipes fillings caused by hydrothermal solutions percolating within limestones. (Jean Feraud)
Ore deposits
of Aveyron : Sites that gave place to an exploitation for the fluorite:
- Valzergues;
- "Le Kaymar" mines (Kaymar and "La Boule").
Other mines or localizations not very interesting for the industrial
aspect:
- Colombies, Compolibat, Pessens, Montpestels...

