George CALAS
Laboratory of Mineralogy - Crystallography
Universities
of Paris 6 and 7 and Institute of Physique of the Earth of Paris, UMR CNRS 7590
4 place Jussieu 75252 Paris Cedex 05
"fluorite
lime is, among all the stony substances, that which lent itself more to the varied
effects of the colouring action of metallic oxides. Also, the illusion which the
resemblance between the colours tends to produce which decorate these various
crystals with that of the stones called gems, it suggested the names of false
ruby, is necessary sapphire, distorts emerald etc, which the former mineralogists
had given him according to the diversity of its colours "
Hauy, Treaty of
Mineralogy (1822)
The CaF2 fluorite, or fluorite, is the natural fluoride most important, presents in particular in many seams often associated with the rocks with granitic composition like in many sedimentary ore deposits. The fluorite is generally presented with crystalline forms expressed very well. This mineral is colourless in a pure state, because it does not absorb visible radiations (the visible spectrum roughly lies between 400 Nm and 750 Nm), being remarkably transparent from ultraviolet ray (125 Nm, i.e. beyond the limit of transparency of the air) to the average infra-red (10 microns), from where its use attends like optical material. The excellent optical quality of the majority of the natural samples of fluorite makes it possible to observe a great diversity of colouring, sometimes associated properties of luminescence under the excitation of ultraviolet rays or x-rays, like at the time of the heating of a sample (thermoluminescence). The great sensitivity of fluorides to the radiations related to the radioactive elements will support the formation of very varied colors, in relation to the presence of coloured centers trapped on various impurities present in the crystal lattice of the fluorite.
THE ORIGIN OF THE COLOR OF THE FLUORITE : A LONG HISTORY...
As the natural fluorites show a large variety of colors, whose remarkable property is to be easily destroyed by heating or even by simple action of daylight, the origin of the colouring of the fluorites was the object for a very long time many discussions. The most varied theories were thus advanced: presence of chromophoric organics, varied impurities, metal colloids and coloured centers, associated or not impurities. The "organic" theory is very old bus the chemical analysis of the natural fluorites very often shows the presence of organic matter. The discolouration of the natural fluorites at high temperature, already highlighted by Saussure, was thus explained by a decomposition of this organic matter. Those in fact are concentrated in fluid inclusions and are not localised within the crystal lattice, and the study of the absorption spectra optical of the natural fluorites made it possible to draw aside this theory definitively. Berthelot, since 1906, had shown that the radioactive materials made it possible to colour in an effective way the fluorites. However, compared to other metal halides alkyl natural, in which the defects related to an irradiation were included/understood as of the years 1960, the defects created by irradiation in the natural fluorites are always associated impurities, which complicates the determination of it. It is in fact the comparison with synthetic crystals which made it possible to determine in a nonambiguous way the origin of the colouring of the natural crystals by showing the influence of certain rare earths and other impurities in the origin of colouring. However certain mechanisms of colouring, as those which are at the origin of the yellow natural fluorites, could not be reproduced in the synthetic crystals.
THE STRUCTURE OF
THE FLUORITE
Traditional example of a cubic lattice centered faces, the fluorite was one of the first minerals of which the structure was determined by William H. Bragg since 1914. This perhaps described structure as a cubic stacking centered faces of Ca2+ ions which delimit tetrahedral cavities occupied by the ions F (Figure 1).
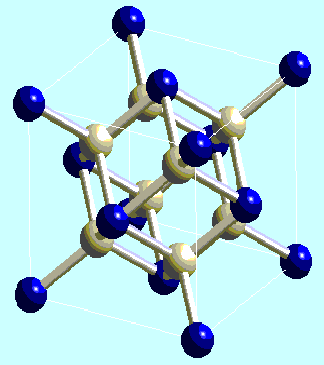
Fig. 1 Structure of the fluorite. The tops of the mesh represent
the atoms of calcium and the atoms of fluorite are in the tetrahedral cavities
of this cubic stacking centered faces.
-- the substitution of rare earths in the network of the fluorite. Calcium
is thus surrounded of 8 neighbors fluorite who form a cubic cavity of big size,
likely to adapt to the impurities like rare earths. The rare earth ions, generally
trivalent, will not be able to replace calcium the network of the fluorite that
if there is a compensation of load which ensures the conservation of the electric
neutrality of the crystal. There are many possibilities to ensure a compensation
of load in a network like that of the fluorite. If the compensation of load is
remote, the initial symmetry of the site occupied by calcium is preserved. One
can thus think that they are the first neighbors contribute to the essence of
the crystal field seen by the cation. On the other hand, a compensation of load
near rare earth inevitably will lower local symmetry. This compensation of load
can be done in the cation site nearest (for example, substitution of an ion calcium
by an ion sodium according to the reaction of following coupled substitution:
2Ca2+=Na+ + TR3+). A compensation of load can also be ensured the level of the
anion environment of rare earth, either by the presence of a fluorite gap or by
the replacement of a part of the ions fluorite by the ions oxygenates. It is interesting
to note that, for a given rare earth, the symmetry of the sites observed in the
natural fluorites will be different from that which is generally given in the
synthetic crystals, and which moreover they varies according to the ore deposits.
--
a simple example of coloured center: the center F When one irradiates an alkyl
metal halide crystal with a radiation of high energy (for example, x-rays or gamma,
electron beam...), one quickly observes a colouring of the sample at the place
where it was irradiated. In the case of sodium chloride, the rock salt, a yellow
colouring appears thus. This colouring is reversible, because if the crystal is
heated, this one is faded by emitting light (thermoluminescence). The origin of
this colouring is in the formation of a center F, which represents a chlorine
gap which trapped an electron at the time of the irradiation, electron torn off
with other defects or an impurity thus forming a positive hole. This lone electron
has various energy levels which will allow transitions to the origin from a specific
absorption from the light. At the time of the heating, the electron will leave
this unstable trap to be trapped on one of the positive holes created at the time
of the irradiation.
In the case of the fluorite, the presence of many impurities
as the rare earths mentioned above will bring the formation of more complex centers
at the same time as it will explain the origin of the diversity of the colors
of the natural crystals. Three different types of colouring can be distinguished:
specific defects associated a rare earth or other impurities, electrons trapped
on a rare earth and calcium colloids. It is however simpler, while remembering
this diversity of origin, successively to examine the principal colourings observed
in the natural fluorites.
THE ORIGIN OF VARIOUS COLORS OBSERVEES IN THE NATURAL FLUORITES
--
the mauve color or violet. It is probably the color most frequently met in the
majority of the ore deposits of fluorites. It is related to the presence of an
intense and broad absorption band located at the medium of the visible spectrum,
in the neighbourhoods of 570 Nm (Figure 2). Absorption bands of lower intensity
are also located in the ultraviolet part of the spectrum, and thus do not influence
the process of colouring; they are related to the presence of other coloured centers
(see low). This intense absorption band is in fact related to the presence of
calcium colloids of size ranging between 30 and 40 Nm, finely dispersed inside
the network of the fluorite. The variability of the colors observed, from intense
blue to the dark mauve, is to be connected to colloids of different size, the
position of the maximum of absorption moving towards larger wavelengths when the
size of colloids increases. The relation with radioactive inclusions is often
highlighted by an irregular distribution of the color, often described like indicating
the presence of pleochroic halations. It is in particular the case of minerals
containing of the uranium which will bring an irradiation of the crystal by rays
alpha on a depth of a few microns. The calcium colloid formation indicates an
important disorganization of the crystal lattice: when their concentration is
important, as in the case of the anthozonite variety which one frequently meets
in the vicinity of the uranium ore deposits, it releases with crushing from mineral
a characteristic fluorite odor.
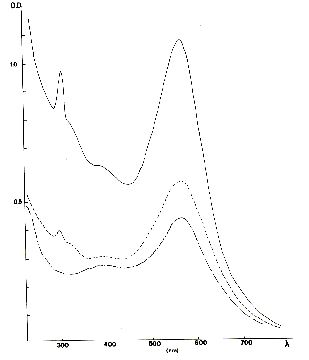
Fig. 2 - optical Absorption spectrum of fluorites violets and mauves. From
top to bottom: Foisches and Fontsantes (Var) and Djebel Kohol (Tunisia). The vertical
axis represents the absorptance (density optical: D.O.) sample. In X-coordinate,
the wavelength out of nanometers.
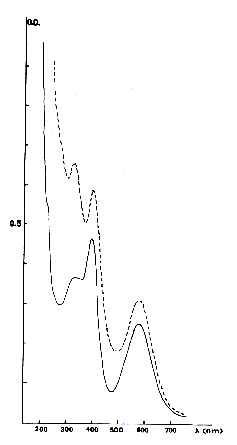
Fig. 3 - optical Absorption spectrum of blue fluorites. In indents, fluorite of
Ponteaumur (Puy de Dome), in full features, fluorite of the mine Moritz (the Vosges).
-- the blue color. When the color is very intense, as in the case of the fluorites
of the type Blue John used in decoration, the origin of colouring is similar with
that of the mauve fluorites described above. There is however another colouring
frequently met, giving crystals of colour much clearer and which is of different
origin. In this case, the optical spectrum is very different from the precedent.
Its complex structure shows the presence of several absorption bands located in
the visible spectrum or the ultraviolet close relation (Figure 3). This color
is easily reproduced by irradiation of the crystals with x-rays Its origin lies
in association between a center F, such as it was described above, and an yttrium
atom in substitution of calcium, giving to this center complexes a symmetry (111).
The frequent presence of zonations shows that the irradiation responsible for
its formation cannot come from the outside of the crystal. The low thickness of
these coloured veins, developed according to faces' of growth, would indicate
an irradiation related to transmitters has. The rays has coming from thorium penetrate
thus of 14 mm in the crystals of fluorite, and the trapping of such impurities
on a face of growth thus makes it possible to return account of these zonations.
A blue fluorescence with violet is sometimes observed in certain fluorites like
those of Derbyshire giving them a particular colour, since the samples can be
green when they are seen by transparency but emit at the same time a blue color
with violet. This property is related to the presence of divalent europium, substituent
calcium, a reduced oxidation step of this rare earth, which translates particularly
reducing conditions of formation at the time of the growth of these fluorites.
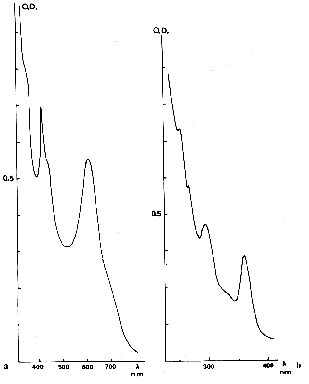
Fig. 4 - optical Absorption spectrum of a green fluorite of
Weardale (Cumberland, England)
-- the green color. It acts a colouring very frequently met in many hydrothermal
fluorites. The optical spectrum is more complex than that which is met in the
case of the other coloured fluorites, with many absorption bands located in the
visible field and close UV. Its origin is related to the presence of a divalent
rare earth, the samarium. As in the case of europium considering higher, this
oxidation step is unusual. In this case however, it does not act of an effect
related to a training in particularly reducing conditions, but of a consequence
of a natural irradiation. When the samarium is trivalent, which corresponds to
its usual oxidation step, the crystal containing this rare earth is colourless.
The action of an irradiation makes it pass to this unstable oxidation step, which
is accompanied by a characteristic green colouring. This colouring can be destroyed
by heating of the crystal, the samarium passing by again again in a trivalent
state.
-- the pink color. This color is characteristic of the fluorites found in the
alpine seams, ore deposits in which the crystals are usually of octahedral facies.
The optical spectrum shows primarily the presence of a broad absorption band centered
towards 485 Nm, with transitions from low intensity, located in the ultraviolet
close relation, thus making it possible only red radiations to be transmitted
by the crystal (Figure 4). The center at the origin of this colouring is composed
of an ion Y3+ associated with a molecular ion O23 -, thus forming a grouping (YO2).
This molecular ion comes from the trapping of a positive hole at the time of the
irradiation two ions close O2 present in impurity in the crystal. This unstable
molecular ion is destroyed by heating, the capture of an electron allowing a dissociation
two ions O2 -. As in the case of colourings seen higher, the coloured center responsible
for pink colouring can be reproduced by crystal irradiation of fluorites which
contain oxygen in impurity.
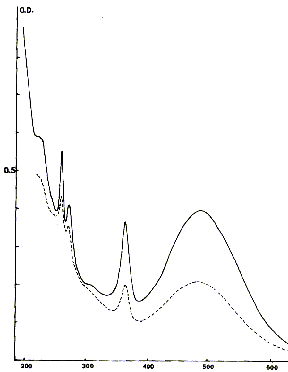
Fig. 5 - optical Absorption spectrum of pink fluorites of the Alps (Periades
and Chamonix)
-- the yellow color. Another colouring characteristic of the fluorites, in particular in the ore deposits of low temperature, the yellow color is related to an absorption spectrum very different from the precedents. Apart from two weak absorption bands located in close UV, the spectrum shows especially the presence of a single absorption band, centered in the zone violet of the spectrum towards 430 Nm as one sees it in the famous fluorites of Valzergues (Aveyron), but also in many other ore deposits like those of Illinois (Figure 5). The presence of a superimposed complex structure, composed of eight lines of low intensity and dependent on the vibrations of the molecular ion at the origin of this colouring indicates that the responsible coloured center consists of a molecular ion O3- which replaces two ions fluorite neighbors. The yellow color is relatively stable thermically, and is destroyed in two days with 320¬įC (Figure 6), to compare with a stability of a few minutes for the blue color at this temperature. Contrary to the majority of other colourings of the fluorite, the yellow color cannot be reproduced in experiments. The process of discolouration thermal of the yellow fluorites is thus irreversible.
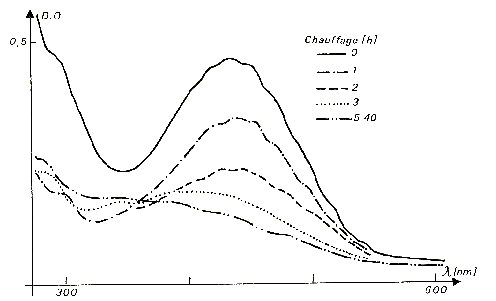
Fig. 6 - optical Absorption spectrum of a yellow fluorite, showing
the influence of an annealing with 320įC (time of annealing in hours).
THE
STABILITY OF THE COLOR OF THE FLUORITES
When the crystals of fluorites are heated, and sometimes simply exposed in the light of the day, they fade in a generally reversible way. The most stable colors thermically are the most intense colors, mauve, purple or blue dark, in relation to the presence of calcium colloids. The least stable are the fluorites of color light blue and their presence can be explained only by one recent irradiation. Other colourings mentioned above are stable at ambient temperature over geological durations: they are thus primary colourings, being able to be created during the growth of the crystal. By studying the kinetics of discolouration of the fluorites, it is possible to use them as maximum indicating temperature beyond which the geological system in which the crystal was sampled could be carried without the crystals being faded. Confrontation with the values of temperature of formation of the crystals, given by fluid inclusions, makes it possible to have several parameters concerning the thermal history of the seam. In the case of the yellow fluorites of Valzergues (Aveyron), formed with 130-140¬įC, one thus could show that the seam could not be carried beyond 220¬įC, temperature above which colouring is destroyed in an irreversible way. The minimum speed of cooling of the seam was of 10-2 ¬įC per annum. One can note that under these conditions, blue colouring observed in many samples of this ore deposit would be thus destroyed in a few days at the temperature of formation of the fluorites, which shows their secondary origin well, dependent on a more recent irradiation. The damage of irradiation in minerals thus makes it possible to give invaluable indications on the history of a sample.